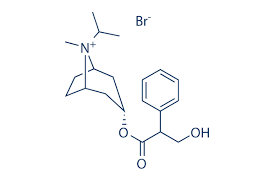
Di Menhydrinate Application: Pharmaceutical Industry
Price: 29 USD / Kilograms
Get Latest Price
Minimum Order Quantity :
100 Kilograms
Product Specifications
| Place of Origin | India |
| Storage | Room Temperature |
| HS Code | 29420090 |
| Ph Level | 6.8-7.3 |
| Molecular Formula | C24H28CIN5O3 |
| Molecular Weight | 469.96 GSM (gm/2) |
| Melting Point | 102 AdegC |
| EINECS No | 208-350-8 |
| Assay | 99% |
| Other Names | Dramamine, Chloranautine |
| CAS No | 523-87-5 |
| Type | Other |
| Grade | Medicine Grade |
| Usage | Antihistamine |
| Purity | 99% |
| Appearance | White crystalline powder |
| Application | Pharmaceutical Industry |
| Smell | No Smell |
| Color | White |
| Form | Powder |
| Supply Ability | 1000 Per Day |
| Delivery Time | 1 Days |
| Packaging Details | 25 kg HDPE drum |
| Certifications | ISO |
Disclaimer
Company Details
Zenita Life Science: Zenita Life Science is India based pharmaceutical company focused on developing, manufacturing and marketing generic pharmaceuticals to the global market. We offer a wide range of products in all the main dosage forms. All the products are manufactured at WHO GMP approved site. Quality Policy : - We at Zenita Life Science are commited to quality in our products and services. We shall strive to achieve professional excellence with continuous improvement in our activities to ensure customer satisfactions. Our extensive product range includes high-quality medicines, Active pharmaceutical Ingredients, Intermediates, Dietary Suppliments, Aroma-Flavour Chemicals, Essential oils and Food Flavours.
Business Type
Exporter, Supplier, Trading Company
Employee Count
10
Establishment
2010
Working Days
Monday To Sunday
GST NO
27AAACZ4571E1Z9
Certification
0
Related Products
Explore Related Categories
Seller Details

GST - 27AAACZ4571E1Z9
Navi Mumbai, Maharashtra

Accepts only Foreign inquiries
Director
Ms Zenita Lifescience Pvt Ltd
Members since
4 Years
Address
B No. 410, Raheja Arcade, Sector 11, CBD Belapur, Navi Mumbai, Maharashtra, 400614, India
active pharmaceutical ingredients in Navi Mumbai
Report incorrect details