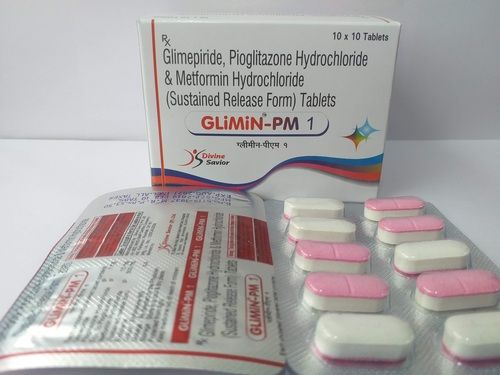
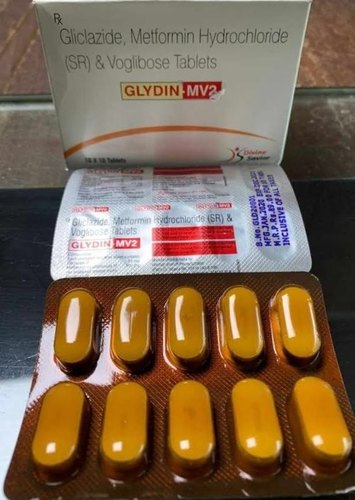
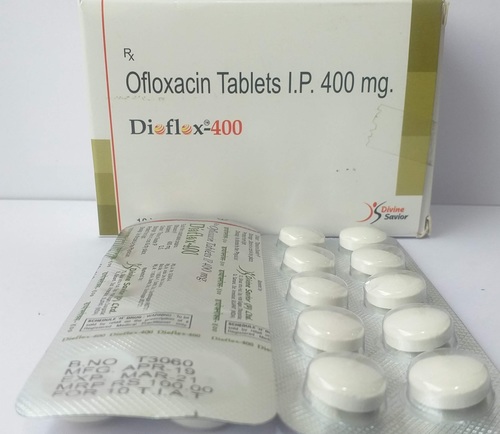
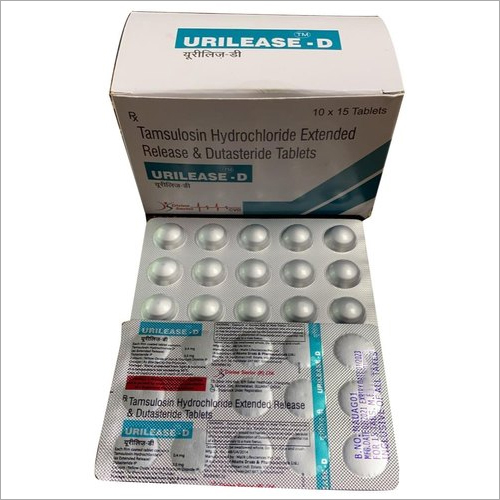
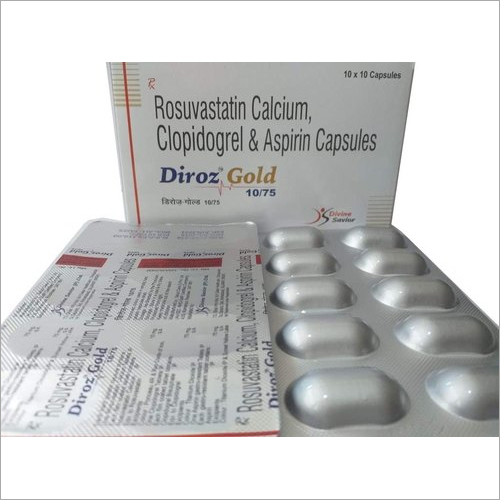
Each Uncoated Dispersible Tablet
Price: 190 INR / Tablet
Get Latest Price
Minimum Order Quantity :
10000 Tablet
Brand Name :
Zyclav 457 Dt Tab
In Stock
Product Specifications
| Medicine Type | Uncoated dispersible tablet |
| Assay | Typically 90%-110% of the active ingredient specification |
| Expiration Date | 24 months from the date of manufacturing |
| Pacakaging (Quantity Per Box) | 100 tablets per box |
| Dosage Form | Tablet |
| Usage | Dissolve in water before administration as per instructions |
| Molecular Formula | Depends on the active pharmaceutical ingredient (API) |
| Storage | Store in a cool dry place away from direct sunlight |
| Appearance | White to off-white round tablets |
| CAS No | Not applicable for finished dosage forms |
| Dosage | Dosage as directed by a physician |
| Salt Composition | Specific API with excipients (details may vary based on formulation) |
| Origin of Medicine | Manufactured in India |
| Grade | Pharmaceutical grade |
| Indication | Used to treat specific medical conditions as prescribed by healthcare professionals |
Company Details
Business Type
Exporter, Manufacturer, Supplier, Wholesaler
Employee Count
100
Establishment
1997
Working Days
Monday To Saturday
GST NO
24AADCD9685J1Z6
Related Products
Explore Related Categories
Seller Details

GST - 24AADCD9685J1Z6
Ahmedabad, Gujarat
Marketing Manager
Mr Kalp Patel
Address
73, Silver Infra Hub, B/h Sakar Health Care, Changodar, Taluka Sanand, Ahmedabad, Gujarat, 382213, India
amoxycillin cloxacillin injection in Ahmedabad
Report incorrect details