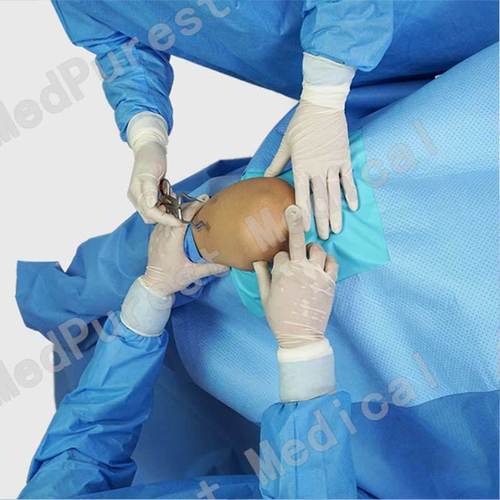
General Surgery Chest Surgical Drapes
Price:
Get Latest Price
In Stock
Product Overview
Key Features
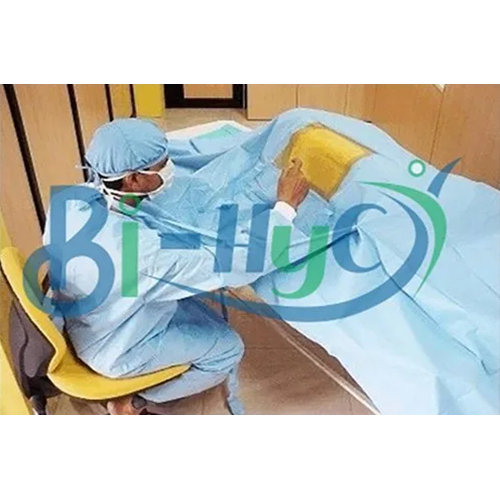
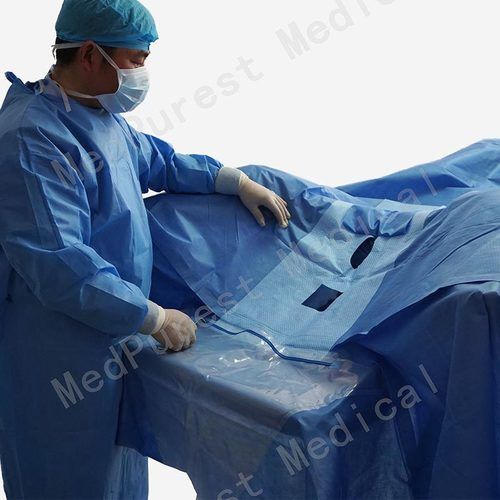
1. Chest Drape, 100 in. x 72 in. x 124 in.
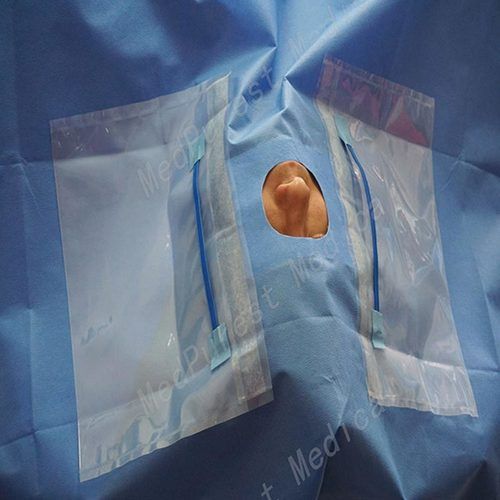
2. 13 in. x 14 in. fenestration.
3. CONTROL* Plus Fabric Reinforcement.
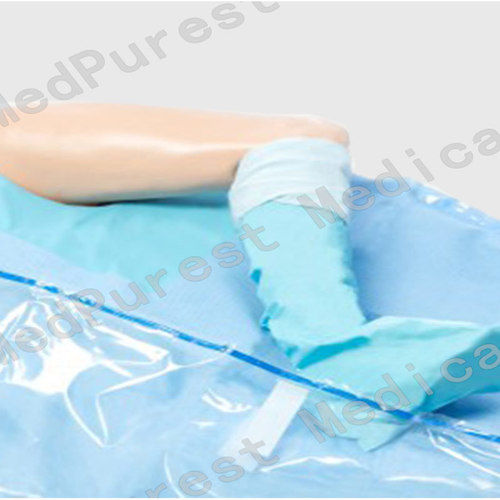
4. Instrument pad.
5. Tube holders.
6. Arm board covers.
Name : General Surgery Chest Drapes
Category : General Surgery
Procedures : Breast, Chest, Biopsy, Plastic Surgery, Reconstruction
Procedure Type : Plastic Surgery
Drape Dimensions : 100 x 72 x 124 in
Fenestration Type : One 13 x 14 in. Rectangular
Fabric : SMS
Fabric Weight : Standard
Line Control System : Fabric Tube Holders
For the full range of general procedures, we've got you covered. From laparotomy and endoscopic drapes to breast, chest and thyroid drapes, MedPurest has adaptable systems that offer maximum flexibility for surgical procedures.
Designed for safety and convenience, MedPurest drapes are developed to protect you and your patients and include the following features:
1. Maximum rating for flame resistance for use around lasers and other electrical instruments.
2. Fabric that's resistant to tearing, strike through and abrasion, while providing secure attachment for line and tubes.
3. Lower lint than other drapes to reduce the risk of airborne bacterial transmission.
4. A choice of fabric reinforcements designed either to reduce instrument slippage or control fluid run-off.
5. Convenient tube and line management systems, including troughs and hook & loops.
Company Details
Focusing on a customer-centric approach, Anhui MedPurest Medical Technology Co.,Ltd has a pan-India presence and caters to a huge consumer base throughout the country. Buy Surgical Dressings & Disposable in bulk from Anhui MedPurest Medical Technology Co.,Ltd at Trade India quality-assured products.
Business Type
Exporter, Manufacturer
Employee Count
200
Establishment
2013
Working Days
Monday To Sunday
Related Products
Explore Related Categories
Seller Details
Shanghai, Shanghai
Mrs Long Shu Shan
Address
null Shanghai, Shanghai, China
surgical drape in Shanghai
Report incorrect details