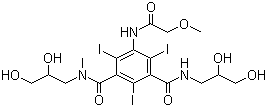
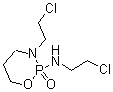
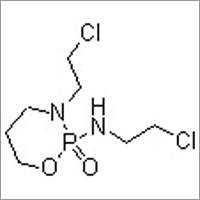
Ifosfamide With Mesna Injection Accuracy: 0.01mm Mm
Price: 4.1 USD / Unit
Get Latest Price
Minimum Order Quantity :
500 units Unit
In Stock
Product Specifications
| FOB Port | Hazira / Nhava Sheva |
| Payment Terms | Telegraphic Transfer (T/T), Cash in Advance (CID), Cash Advance (CA) |
| Supply Ability | 5000 Per Week |
| Delivery Time | 10 Days |
| Packaging Details | 1 Vial in a box |
| Main Export Market(s) | Australia, North America, South America, Eastern Europe, Western Europe, Middle East, Africa, Asia, Central America |
| Certifications | WHO-GMP/GMP/COA/As Required By Client |
Product Overview
Key Features
Mesna is a medication used in those taking ifosfamide to decrease the risk of bleeding from the bladder.
USES OFIFOSFAMIDE:
-Testicular cancer
-Soft tissue sarcoma
-Osteosarcoma
-Bladder cancer
-Small cell lung cancer
-Cervical cancer
-Ovarian cancer
USES OFMESNA:
Incidence of hemorrhagic cystitis
Haematuria
As mucolytic agent
AVAILABLE STRENGTH:
MECHANISM OF ACTION:
Ifosfamide:
It works by disrupting the duplication of DNA and the creation of RNA, thus inhibiting the formation of cancer cells.
MESNA:
Mesna reduces the toxicity of urotoxic compounds that may form after chemotherapy administration, by reduces the urotoxic compounds to unharmful metabolites.
SIDE EFFECTS:
Ifosfamide:
Hair loss
Vomiting
Blood in the urine
Infections
Kidney problems
MESNA:
Allergic reactions
PRECAUTION AND HOW TO USE:
The drug should be administered under the supervision of an experienced cancer chemotherapy physician.
Bone marrow suppression may occur.
Confusion and coma due to CNS toxicity have been associated with therapy. Discontinue therapy if it occurs.
Avoid in pregnancy.
Use caution in renal impairment.
Disclaimer
Company Details
SALVAVIDAS PHARMACEUTICAL PVT LTD is a reputed and fast growing Manufacturing Company in Pharmaceutical & Healthcare Industry for over a decade, based in Surat, Gujarat, India. SALVAVIDAS PHARMACEUTICAL is a leading and well established name in the field as Manufacturer, Exporter and Supplier of Pharmaceutical Finished Formulation such As Tablets, Capsules, Injections, Vials, Ampoules, Creams, Ointments, Drops, Syrups, Suspensions, Lotions, Nutraceuticals, Active pharmaceutical Ingredients (API & Bulk Drugs) and Surgical Products across the globe. All our Products are manufactured in WHO GMP, ISO 9001 Approved Manufacturing Facilities. We have a robust product portfolio comprising of Medicines spread over major therapeutic areas particularly Antibacterial, Antimalarial, Antiviral, Antifungal,Antitubercular, Erectile dysfunction, Antidepressants, Antipsychotic, NSAID, Painkiller , Analgesic, Anesthetic, Antiallergic, Antidiabetic, Antiemetic, Antiasthmatic, Antacid, Antibiotic, Cardiovascular, Antihypertensive, Dermatological and many more. The company is into manufacturing and exporting of Pharmaceutical Products under almost all dosage forms and categories consisting more than 380 products. One of our specializations is Contract Manufacturing and 3rd Party Manufacturing where we ensure we manufacture the required pharmaceutical drugs and make it easily accessible to the market. Whether our client would like us to modify or enhance an existing product, create a new drug or increase the present manufacturing capacity of particular medicine, our team of professionals combined with our infrastructure is always ready for it.
Business Type
Exporter, Manufacturer, Distributor, Supplier, Trading Company
Employee Count
50
Establishment
2015
Working Days
Monday To Saturday
GST NO
24AAWCS4912L1ZS
Payment Mode
Letter of Credit (L/C)
Certification
ISO 9001:2008
Related Products
Seller Details

GST - 24AAWCS4912L1ZS
Surat, Gujarat

Accepts only Foreign inquiries
Director
Mr Viral Dhaduk
Members since
11 Years
Address
1st FLOOR, OFFICE - 102, SHUBH SQUARE, PATEL WADI - 3, OPP. SHIVANJALI ROW HOUSE, Lal Darwaja, Surat, Gujarat, 395004, India
mesna in Surat
Report incorrect details