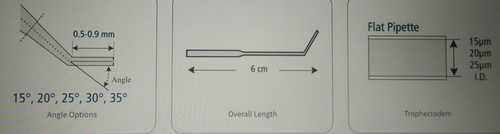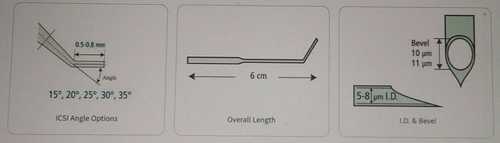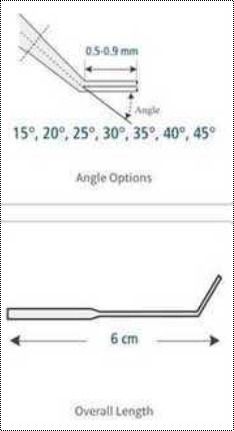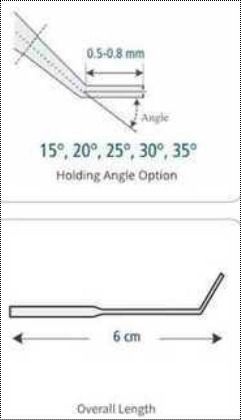
Lightweight And Portable Trophectoderm Biopsy (Bevelled) Micropipette
Price:
Get Latest Price
In Stock
Product Specifications
| Color | As shown in the image |
| Usage | For Laboratory |
| Size | Customized |
| Product Type | Micropipettes |
| Application | Laboratory |
| Supply Ability | 200 Per Month |
| Delivery Time | 1 |
| Sample Policy | Contact us for information regarding our sample policy |
| Packaging Details | > Each micropipette is carefully packed on its own and guaranteed to be sterile.> Measurement tolerance: A+-5%> Taper length: 900 micrometers |
| Main Export Market(s) | Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa |
| Main Domestic Market | All India |
| Certifications | Monash Biotech operates in compliance with the current Good Manufacturing Practices (CGMP's), additionally it adheres to ISO 13485:2016 regulations and Central Drugs Standard Control Organization (CDSCO) Certificate. |
Product Overview
Key Features
Explore the precision and reliability that Monash Biotech brings to Trophectoderm Biopsy procedures.
Company Details
Monash Biotech, Founded on a core philosophy of delivering unparalleled quality across products and services, Monash Biotech stands as a beacon of excellence in the global micromanipulation industry. Our commitment is to serve as a steadfast and reliable partner to professionals and markets alike, leveraging our inherent strengths for sustainable growth.
At the heart of Monash Biotech's esteemed reputation as a leading medical device manufacturer is our unwavering dedication to legal, moral, and ethical integrity. This foundation compels us to uphold strict compliance with both the law and our rigorous standards of business conduct. Our collective endeavor is to embody and rigorously enforce our business code, uphold our quality policy, and achieve our shared vision of delivering exemplary customer service to our esteemed clientele, vendor partners, and manufacturing suppliers worldwide.
Monash Biotech's product line is crafted in strict accordance with CE standards, current Good Manufacturing Practices (CGMPs), and ISO 13485:2003 regulations. Our production protocols and quality control procedures are meticulously designed to ensure the highest standards of product integrity.
Furthermore, our offerings are certified through Mouse Embryo Test and Endotoxin tests, guaranteeing that every product is developed and quality-assured under the expert supervision of scientists renowned for their decades of contribution to Assisted Reproductive Technology (A.R.T.). For embryologists and medical professionals, Monash Biotech signifies more than a supplier âA A we are a partner dedicated to advancing the frontiers of reproductive science with integrity, precision, and unwavering quality.
Business Type
Exporter, Manufacturer, Supplier
Employee Count
9
Establishment
2018
Working Days
Monday To Saturday
GST NO
24AAMCM0456F1ZM
Payment Mode
Cash Advance (CA)
Certification
ISO 13485:2016
Related Products
Explore Related Categories
Seller Details

GST - 24AAMCM0456F1ZM
Vadodara, Gujarat
Director
Mr Shobhan R. Vyas
Address
78/2/4 GIDC, Makarpura, Vadodara, Gujarat, 390010, India
micropipettes in Vadodara
Report incorrect details