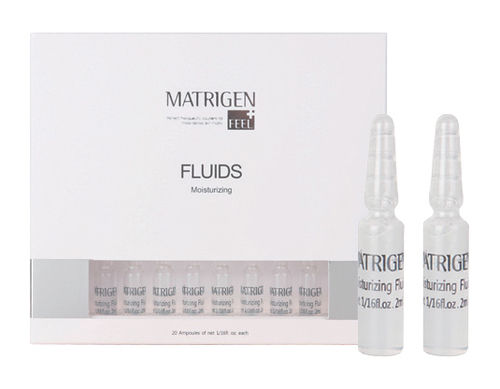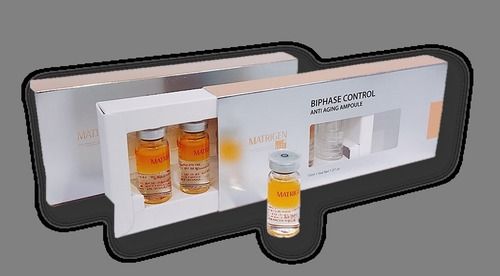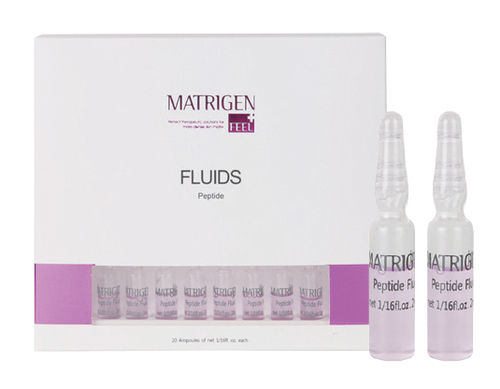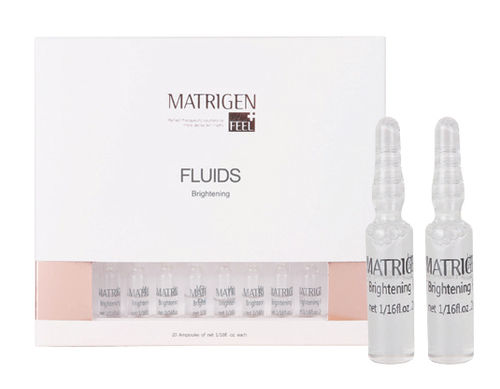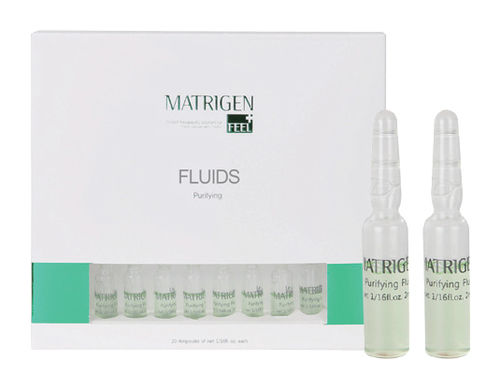
Safe To Use Matrigen Cell Repair Fluid
Price:
Get Latest Price
In Stock
Product Specifications
| Use | Face, Skin care |
| Form | Other |
| Quality | Safe To Use |
| Shelf Life | 2 years & 6 month Years |
| FOB Port | Incheon Airpor or Incheon port or Pusan Port |
| Supply Ability | 25000 Per Day |
| Sample Available | Yes |
| Sample Policy | Contact us for information regarding our sample policy |
| Packaging Details | (490*400*260)mm 7.8 kg / Carton |
| Main Export Market(s) | Asia |
| Certifications | ISO 22716 |
Product Overview
Key Features
- Strengthen skin protecting power from the external environments
- Stimulate body circulation, accelerate cell regeneration, Brightening effect
- Relieving scratched skin tissue by accelerating cell regeneration
Function: Plant stem extract Accelerates skin cell production, skin regeneration and Wrinkle improvement
Effects:
- Ginseng extract accelerates collagen production
- preventing wrinkles
- improves skin trouble with sterilization
- calming & moisturizing
* MAIN INGREDIENTS
Ginseng callose extract:
- Accelerate collagen production
- Wrinkle improvement
Damask rose callose extract:
- Skin brightening, improve skin immunity
- Brightening by restraining tyrosinase
- Calming, Improving skin trouble by bisabolol effects
Greentea callose extract: Antioxidation, bisabolol effects
High-enriched bioactive substance is effective on anti corrugation and whitening
Aloe Vera leaf extract
Gives moisture to sensitive & dehydrated skin
Disclaimer
Company Details
Business Type
Exporter, Manufacturer, Supplier
Employee Count
25
Establishment
1993
Working Days
Monday To Friday
Payment Mode
Telegraphic Transfer (T/T)
Certification
ISO 22716 : 2007
Related Products
Explore Related Categories
Seller Details
Incheon, Incheon
Overseas Sales Manager
Mr. Alex Yang
Address
26-2, Janggogae-ro, 243Beon-gil, Seo-gu, Incheon-si, South Korea, Incheon, Incheon, 22827, Korea South
skin care cosmetics in Incheon
Report incorrect details