Phospho Tungstic Acid - Application: Lab Chemicals
Price: 7800.00 INR / Kilograms
(7800.00 INR + 0% GST)
Get Latest Price
Minimum Pack Size :
8
In Stock
Product Specifications
| Application | Lab Chemicals |
| Storage | Room Temperature |
| CAS No | 1343-93-7 |
| Grade | Extra Pure |
| HS Code | 2811 |
| Physical Form | Powder |
| Usage | CatalystIn common with the other heteropolyacids phosphotungstic acid is a catalyst and its high acidity and thermal stability make it a catalyst of choice according to some researchers.[13] It is in solution as a homogeneous catalyst, and as a heterogeneous catalyst "supported" on a substrate e.g. alumina, silica. Some acid catalysed reactions include:the homogeneous catalysis of the hydrolysis of propene to give 2-propanolthe homogeneous catalysis of the Prins reactionthe heterogeneous catalysis of the dehydration of 2-propanol to propene and methanol to hydrocarbons.Dyeing and pigmentsPhosphotungstic acid has been used to precipitate different types of dyes as "lakes".[14] Examples are basic dyes and triphenylmethane dyes, e.g. pararosaniline derivatives.HistologyPhosphotungstic acid is used in histology for staining specimens, as a component of phosphotungstic acid haematoxylin, PTAH, and aEURoetrichromeaEUR reagents, and as a negative stain for imaging by a transmission electron microscope.Phosphotungstic acid haematoxylin (PTAH)Mallory described the reagent now generally known as PTAH in 1897.[16] PTAH stains tissues either reddish brown or blue depending on their type. This property of simultaneously staining two different colours is different from other haematoxylin reagents e.g. alum-haematoxylin. The role of phosphotungstic acid and the mechanism of staining is not fully understood. Interestingly the active component of haematoxylin is the oxidised form, haematin, although this rarely acknowledged in the literature which refer to haematoxylin staining. Phosphotungstic acid forms a lake with haematin.[17] The make aEUR"up of the reagent is uncertain, examination of a year old sample showed there to be three coloured components, blue, red and yellow.[18] These were not identified. Some investigations of aEURoemodelaEUR systems, reacting various compounds such as amino acids, purines, pyrimidines and amines with PTAH show that they give rise to different colours.Trichrome reagentsIn these reagents two or three basic dyes are used with phosphotungstic acid, in either a one step or multi-stage procedure. These reagents colour different tissue types different colours. Again the mechanism of staining is not fully understood. Some explanations include the proposal that phosphotungstic acid acts as a mordant to bind the dye to the tissue[20] or that alternatively it binds to tissue blocking it to dye molecules.Negative stainingAdsorption onto tissue or the surface of viruses and its electron density are the bases of phosphotungstic acids action as a negative stain. This electron density arises from the presence of the 12 tungsten atoms which each have an atomic number of 74. The mechanism of the adsorption onto tissue has been proposed as being electrostatic rather than involving hydrogen bonding, as adsorption is not affected by pH.AnalysisThe potassium salt is only slightly soluble, unlike most other phosphotungstate salts, and has been proposed as a method for the gravimetric analysis of potassium.Precipitation of proteinsIn a number of analytical procedures one of the roles of phosphotungstic acid is to precipitate out proteins. It has been termed a "universal" precipitant for polar proteins.[23] Further studies showed that no precipitation occurred with I+--amino groups but did occur with guanidino, Iu-amino and imidazole groups.MedicinalVery little work appears to have been carried out in this area. One example relates to liver necrosis in rats.Composite proton exchange membranesThe heteropoly acids, including phosphotungstic acid, are being investigated as materials in composite proton exchange membranes, such as Nafion. The interest lies in the potential of these composite materials in the manufacture of fuel cells as they have improved operating characteristics |
| Shape | Crystal |
| Shelf Life | 5 Years |
| Solubility | water soluble |
| Taste | Odorless |
| Melting Point | 89 AdegC |
| Product Type | Powder |
| Molecular Weight | 2,880.2 g/mol Kilograms (kg) |
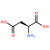
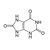
| Molecular Formula | H3PW12O40 |
| Structural Formula | H3PW12O40 |
| Properties | Phosphotungstic acid can be prepared by the reaction of sodium tungstate, Na2WO4.2H2O, with phosphoric acid, H3PO4, acidified with hydrochloric acid, HCl.[2]Phosphotungstic acid solutions decompose as the pH is increased. A step-wise decomposition has been determined and the approximate compositions at various pH values are as follows:[8]pH principal components1.0 [PW12O40]3a^'2.2 [PW12O40]3a^', [P2W21O71]6a^', [PW11O39]7a^'3.5 [PW12O40]3a^', [P2W21O71]6a^', [PW11O39]7a^', [P2W18O62]6a^', [P2W19O67]10a^'5.4 [P2W21O71]6a^', [PW11O39]7a^', [P2W18O62]6a^'7.3 [PW9O34]9a^'8.3 PO43a^', WO42a^'The species [PW11O39]7a^' is a lacunary, or defective Keggin ion. The [P2W18O62]6a^' has a Dawson structure. At pH less than 8, the presence of ethanol or acetone stabilises the anion, [PW12O40]3a^', reducing decomposition.[8]Tungstophosphoric acid is thermally stable up to 400 AdegC, and is more stable than the analogous silicotungstic acid, H4SiW12O40.[9]Large quantities of polar molecules such as pyridine are absorbed into the bulk phase and not simply on the surface. Solid state NMR studies of ethanol absorbed in the bulk phase show that both protonated dimers, ((C2H5OH)2H+) and monomers, (C2H5OH2+) are present.Phosphotungstic acid is less sensitive to reduction than phosphomolybdic acid. Reduction with uric acid or iron(II) sulfate produces a brown coloured compound. the related silicotungstic acid when reduced forms a similar brown compound where one of the four W3 units in the Keggin structure becomes a metal-metal bonded cluster of three edge shared W(IV) octahedra.[10]Phosphotungstic acid is the strongest of heteropolyacids. Its conjugate base is the PW12O403a^' anion.[11] Its acidity in acetic acid has been investigated and shows that the three protons dissociate independently rather than sequentially, and the acid sites are of the same strength.[12] One estimate of the acidity is that the solid has an acidity stronger than H0 =a^'13.16,[9] which would qualify the compound as a superacid. This acidic strength means that even at low pH the acid is fully dissociated. |
| Purity | 99%min |
| Poisonous | NO |
| Other Names | Phospho Tungstic Acid |
| FOB Port | Mumbai |
| Payment Terms | Cash on Delivery (COD), Cash Against Delivery (CAD), Cash in Advance (CID), Cheque, Cash Advance (CA) |
| Supply Ability | 1000 Per Day |
| Delivery Time | 1 Week |
| Sample Available | Yes |
| Sample Policy | Within a certain price range free samples are available |
| Packaging Details | with 25 kg drum packing |
| Main Export Market(s) | Eastern Europe, Western Europe, Central America, Middle East, South America, Asia, North America, Australia, Africa |
| Main Domestic Market | All India |
| Returnable | No |
| Moq | 8 |
| Unit Type | Piece/Pieces |
| Price Type | fixed |
| Price | 7800.00 INR (Approx.) |
| Stock Quantity | 40 |
| Product Unit | 8 Piece/Pieces |
| Brand Name | KAROLINSKA |
| Mop | 8 |
| Currency | INR |
| Minimum Order Quantity | 8 |
| Minimum Ordered Packs | 8 |
| GSTIN | 0% |
Company Details
Focusing on a customer-centric approach, Evans Chem india Pvt. Ltd. has a pan-India presence and caters to a huge consumer base throughout the country. Buy Healthcare & Hygiene Products in bulk from Evans Chem india Pvt. Ltd. at Trade India quality-assured products.
Business Type
Exporter, Manufacturer, Supplier, Producer
Employee Count
12
Establishment
2003
Working Days
Monday To Saturday
GST NO
27AABCE3201D1ZE
Payment Mode
Cash Against Delivery (CAD)
Related Products
Explore Related Categories
Seller Details

GST - 27AABCE3201D1ZE
Mumbai, Maharashtra
Proprietor
Mrs Dhara
Members since
11 Years
Address
401, Aashiya Narsi Chaya, 18 Swastik Soc, North South Rd 2, Vile Parle West, Mumbai, Maharashtra, 400056, India
Report incorrect details