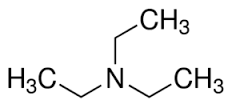
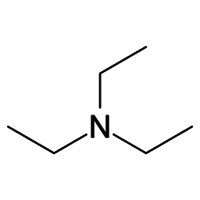
Triethylamine By Mehta Chemicals
Price: 126 INR / Barrel
Get Latest Price
Minimum Order Quantity :
150kg Barrel
In Stock
Product Specifications
| Physical Form | Liquid |
| Usage | Used in pharma industries |
| Appearance | white |
| Grade | pharma |
| Purity | 99.9% |
| Density | 0.72 |
| Supply Ability | 25 barrels Per Day |
| Delivery Time | 2 Days |
| Sample Policy | Contact us for information regarding our sample policy |
| Packaging Details | 150 kg drum packing |
| Main Domestic Market | All India |
Product Overview
Key Features
Triethylamine is prepared by the alkylation of ammonia with ethanol:[8]
NH3 + 3 C2H5OH a N(C2H5)3 + 3 H2O
The pKa of protonated triethylamine is 10.75,[3] and it can be used to prepare buffer solutions at that pH. The hydrochloride salt, triethylamine hydrochloride (triethylammonium chloride), is a colorless, odorless, and hygroscopic powder, which decomposes when heated to 261 A C.
Laboratory samples of triethylamine can be purified by distilling from calcium hydride.
Physical Properties
The chemical formula for triethylamine is C6 H1 N5, and its molecular weight is 101.19 g/mol. (3)
Triethylamine occurs as a colorless flammable liquid that is slightly soluble in water. (1,3,5)
Triethylamine has a strong fishy ammonia-like odor, with an odor threshold of 0.48 parts per million
(ppm). (1,3,5)
The vapor pressure for triethylamine is 400 mm Hg at 31.5 A C, and its log octanol/water partition
coefficient (log K
ow
) is 1.45. (1)
Conversion Factors:
To convert concentrations in air (at 25 A C) from ppm to mg/m
3
: mg/m
3
= (ppm) A (molecular weight of the
compound)/(24.45). For triethylamine: 1 ppm = 4.14 mg/m
3
Applications
Triethylamine is commonly employed in organic synthesis as a base. For example, it is commonly used as a base during the preparation of esters and amides from acyl chlorides.[10] Such reactions lead to the production of hydrogen chloride which combines with triethylamine to form the salt triethylamine hydrochloride, commonly called triethylammonium chloride. This reaction removes the hydrogen chloride from the reaction mixture, which can be required for these reactions to proceed to completion (R, R' = alkyl, aryl):
R2NH + R'C(O)Cl + Et3N a R'C(O)NR2 + Et3NH+Cla
Like other tertiary amines, it catalyzes the formation of urethane foams and epoxy resins. It is also useful in dehydrohalogenation reactions and Swern oxidations.
Triethylamine is readily alkylated to give the corresponding quaternary ammonium salt:
RI + Et3N a Et3NR+Ia
Triethylamine is mainly used in the production of quaternary ammonium compounds for textile auxiliaries and quaternary ammonium salts of dyes. It is also a catalyst and acid neutralizer for condensation reactions and is useful as an intermediate for manufacturing medicines, pesticides and other chemicals.
Triethylamine salts like any other tertiary ammonium salts are used as an ion-interaction reagent in ion interaction chromatography, due to their amphiphilic properties. Unlike quaternary ammonium salts, tertiary ammonium salts are much more volatile, therefore mass spectrometry can be used while performing analysis.
Niche uses
Triethylamine is used to give salts of various carboxylic acid-containing pesticides, e.g. Triclopyr and 2,4-dichlorophenoxyacetic acid[citation needed]
Triethylamine is the active ingredient in FlyNap, a product for anesthetizing Drosophila melanogaster.[citation needed] Triethylamine is used in mosquito and vector control labs to anesthetize mosquitoes. This is done to preserve any viral material that might be present during species identification.
Also, the bicarbonate salt of triethylamine (often abbreviated TEAB, triethylammonium bicarbonate) is useful in reverse phase chromatography, often in a gradient to purify nucleotides and other biomolecules.[citation needed]
Triethylamine was found during the early 1940s to be hypergolic in combination with nitric acid, and was considered a possible propellant.
Company Details
Focusing on a customer-centric approach, MEHTA CHEMICALS has a pan-India presence and caters to a huge consumer base throughout the country. Buy Chemical Supplies in bulk from MEHTA CHEMICALS at Trade India quality-assured products.
Business Type
Manufacturer, Supplier, Trading Company, Wholesaler
Employee Count
10
Establishment
2013
Working Days
Monday To Sunday
GST NO
27BEPPM0929G2Z8
Related Products
Seller Details

GST - 27BEPPM0929G2Z8
Mira Bhayandar, Maharashtra
Proprietor
Mr. Mahendra Mehta
Address
1010, Janki Heritage,150 Feet Road, Near Shree Nidhi Hotel, Opp Maxus Mall, Bhayander (W) , Mira Bhayandar, Maharashtra, 401101, India
triethylamine in Mira Bhayandar
Report incorrect details