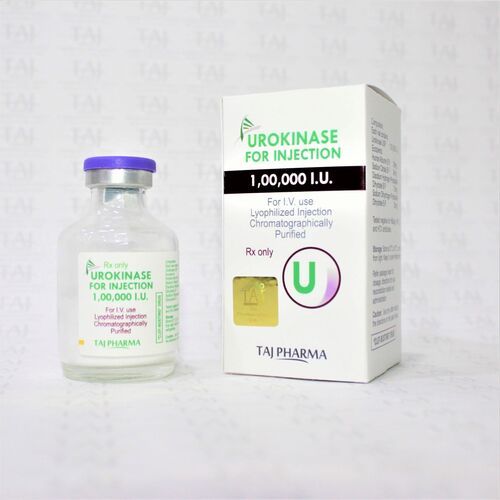

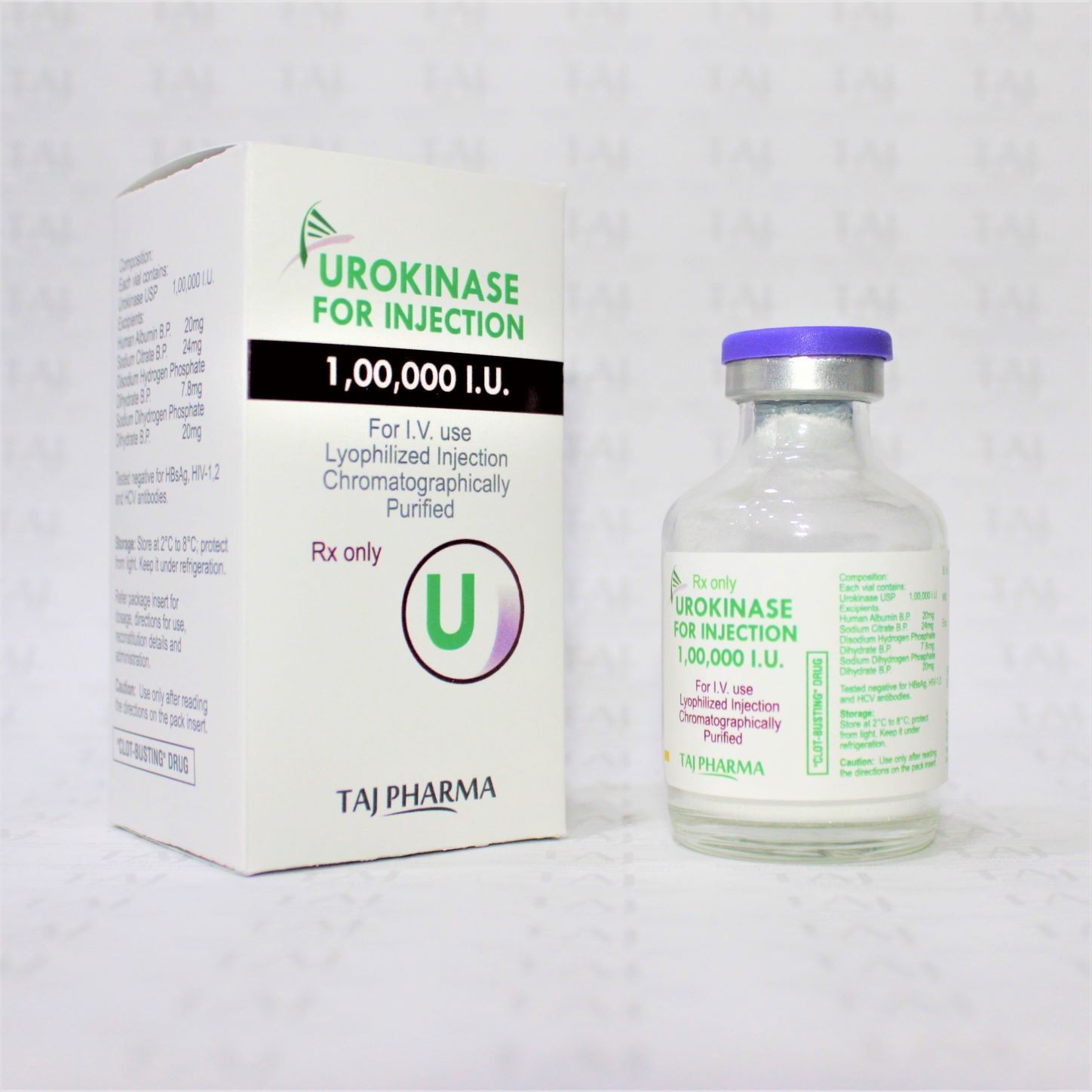

Urokinase For Injection 100000 Iu Expiration Date: 2 Years
Price: 4295.20 INR / Bottle
(3835.00 + 12% GST)
Get Latest Price
Weight :
5.00
1 Pack Contains :
1
Minimum Pack Size :
1000
In Stock
Product Specifications
| Storage | Store Below 25 degree C. Protect from light. Do not freeze. |
| Appearance | Lyophilized Powder for Injection |
| Expiration Date | 2 Years |
| Dosage | Injection |
| Grade | Pharmaceutical |
| Usage | Use to treat large blood clots formed in the lungs |
| Assay | 100% |
| Medicine Type | Anti-coagulant |
| FOB Port | Gujarat |
| Supply Ability | 500000 Per Month |
| Sample Policy | Free samples are available |
| Certifications | WHO-GMP, GMP, GLP, PICS, EU GMP, US FDA Regulatory Documents: a) Certificate of Analysis (COA) b) Method of Analysis (MOA) c) Stability Data (Accelerated stability / Long term stability / Zone 4b) d) CTD Dossier / ACTD Dossiers / eCTD Dossiers e) Certificate of Pharmaceuticals Product (COPP) f) Free Sale Certificate (FSC)s |
| Delivery Time | 30 Days |
| Main Export Market(s) | North America |
| Main Export Market(s) | South America |
| Main Export Market(s) | Eastern Europe |
| Main Export Market(s) | Western Europe |
| Main Export Market(s) | Middle East |
| Main Export Market(s) | Asia |
| Main Export Market(s) | Australia |
| Main Export Market(s) | Central America |
| Main Export Market(s) | Africa |
| Payment Terms | Letter of Credit at Sight (Sight L/C) |
| Payment Terms | Cheque |
| Payment Terms | Letter of Credit (L/C) |
| Sample Available | 1 |
| Main Domestic Market | All India |
| Packaging Details | 1 Vial + WFI Tray |
| Weight | 5.00 |
| Gst | 12 |
| Unit | 1000 |
| Unit Type | Bottle/Bottles |
| Moq | 1000 |
| Mrp | 4300.00 |
| Price | 3835.00 |
| Brand Name | Urokinase for Injection 100000 IU |
| Currency | INR |
| Packsize | 1 |
| Mop | 1000 |
| GSTIN | 12% |
Disclaimer
Company Details
Taj Pharmaceuticals Limited manufactures and distributes medicines. Our products include prescription solutions, lyophilized injections, critical care medicines, lifesaving drugs, anti-cancer drugs, veterinary products, consumer brands, and CNS drugs. It focuses on medicines of anemia, anxiety disorders, cancer/oncology, ear infections, heart failure, hepatitis, HIV/AIDS, influenza, non-Hodgkins lymphoma, obesity, osteoporosis, Parkinsons disease, pneumonia, transplantation, and common diseases. Taj Pharmaceuticals Limited was founded in 2004 and is headquartered in Mumbai, India. Product Specialization : Tablets Capsules, Liquid Injection, Lyophilized Injection, Dry Powder Injection, Oral Liquids, External Preparation ( Cream/Ointment/Jelly), Prefilled Syringe (PFS), Suppositories, Cytotoxic drugs (Oncology), Small Volume Parenterals (SVP), Soft Gelatin. The company is one of the top 10 generic pharmaceutical firms in India and the worlds leader in generics. Taj Pharma India is a market leader in therapeutic fields like Critical care, transplantation, virology, and cancer. Taj Pharma generic medicines and contract manufacturing businesses work well together and our knowledge of the bio-similar generic drugs enable us to create individualized therapeutic strategies and integrated healthcare solutions. We currently sell to consumers in more than 40 countries and territories more than 500 branded and 4600 generic formulations. Taj Pharma guarantees to maintain all ethical standards as a generic medicine manufacturers in the Indian pharma industry to give our customers the highest level of product satisfaction. Our strict production procedures have helped us reach a respectable position in the industry. Our team will constantly make every effort for a greater understanding of your demand for modern generic medicines with every conscious endeavor to offer a high-quality Pharma product to clients and people. Taj Pharma plans to make an effort to widen our assessments, so that we can better serve common people through our diligent efforts. Our offered products include Tablets, Capsules, Anti-Cancer, Lyophilized freeze dried Injection, Inhalers, New Biosimilars, Hormones, Pre-Filled-Syringes (PFS), Nutraceuticals, Suppositories, Beta Lactam Antibiotics range to name a few of them. Our New Sarigam GIDC, Vapi - EU GMP, PICs Formulations Manufacturing Plant include Sections: Tablets 120million per Month Capsules 40million per Month Dry Powders Blended / Granules/Pellets 20million per Month Effervescent Tablets 20million per Month Oral Jelly 60million per Month Cream & Ointments 40million per Month
Business Type
Exporter, Manufacturer, Supplier, Producer
Employee Count
1000
Establishment
2004
Working Days
Monday To Saturday
GST NO
24AACCT1480C1ZT
Payment Mode
Online Payments (NEFT/RTGS/IMPS)
Certification
FDA / WHO-GMP / EU GMP / PICs / ISO 9001:2015
Related Products
Explore Related Categories
Seller Details

GST - 24AACCT1480C1ZT
Ahmedabad, Gujarat
Director
Mr Shantanu Kumar Singh
Address
5TH FLOOR, 505, SAKAR IV, ASHRAM ROAD, Ahmedabad, Gujarat, 380006, India
generic pharmaceutical drugs in Ahmedabad
Report incorrect details