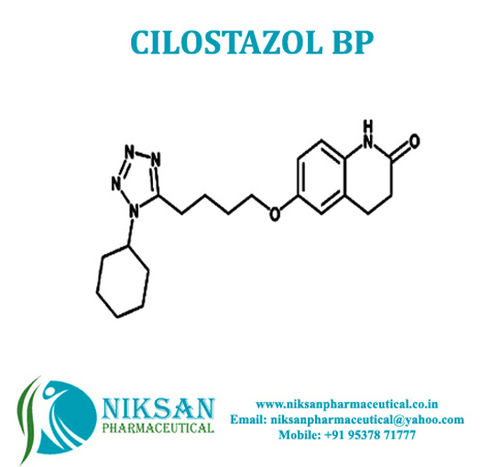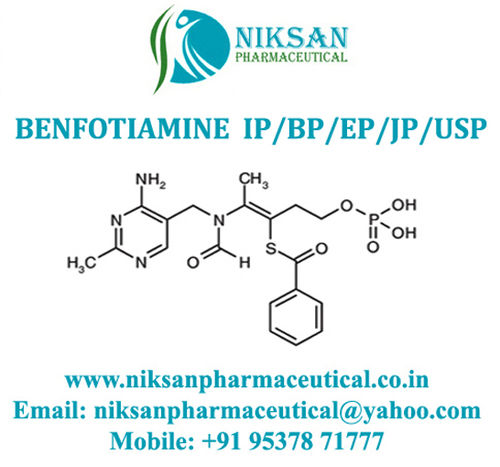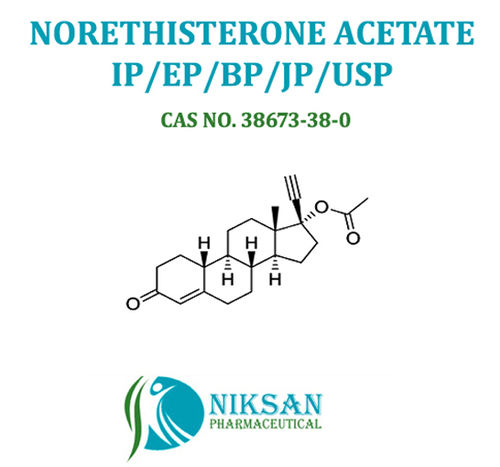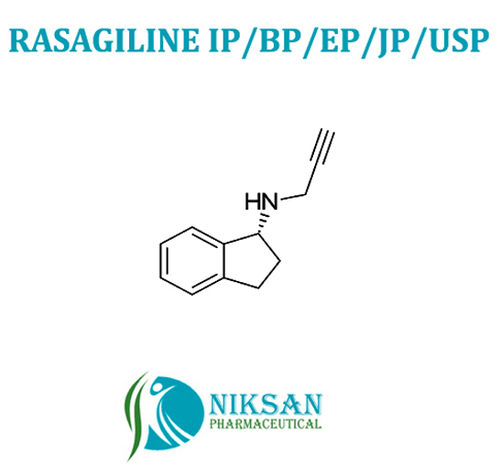
Voglibose - Cas No: 83480-29-9, C10h21no7 Molecular Formula | Anti-diabetic Medicine For Type Ii Diabetes Management, Improves Blood Sugar Control
Price: 520000 INR / Gram
Get Latest Price
Minimum Order Quantity :
50 Gram
In Stock
Product Specifications
| Application | Voglibose is used to improve blood sugar control in adults with type II diabetes. It is used in the treatment of type II diabetes mellitus. Voglibose is an anti-diabetic medicine. It inhibits the intestinal enzymes responsible for breaking complex sugars like glucose. |
| CAS No | 83480-29-9 |
| Molecular Formula | C10H21NO7 |
| Formulations Type | Medicine Raw Materials |
| Formulations Form | Powder |
| Treatments & Functions | type II Diabetes Mellitus |
| Dosage Guidelines | Voglibose should taken by mouth with or without food. Follow all directions on your prescription label or as your doctor recommends you. |
| Storage Instructions | Store in Cool |
| FOB Port | SAHAR AIR CARGO |
| Payment Terms | Cash on Delivery (COD), Paypal, Letter of Credit (L/C), Western Union, Letter of Credit at Sight (Sight L/C), Cash Against Delivery (CAD), Telegraphic Transfer (T/T), Others, Delivery Point (DP), Days after Acceptance (DA), Cash in Advance (CID), Cheque, Cash Advance (CA) |
| Supply Ability | 100 Per Month |
| Delivery Time | 15 Days |
| Sample Policy | Within a certain price range free samples are available |
| Packaging Details | Double LDPE Liners in HDPE carboys |
| Main Export Market(s) | Western Europe, Australia, Eastern Europe, Middle East, Central America, South America, Asia, North America, Africa |
| Main Domestic Market | Himachal Pradesh, Andaman and Nicobar Islands, Nagaland, Uttarakhand, Daman and Diu, Dadra and Nagar Haveli, Lakshadweep, South India, East India, West India, Andhra Pradesh, Assam, Arunachal Pradesh, Chandigarh, Goa, Haryana, Jammu and Kashmir, Jharkhand, Karnataka, Madhya Pradesh, Mizoram, Meghalaya, Manipur, Punjab, Pondicherry, Rajasthan, Sikkim, Tamil Nadu, Tripura, West Bengal, Maharashtra, Delhi, Gujarat, North India, Bihar, Telangana, Kerala, Central India, Odisha, Chhattisgarh, Uttar Pradesh, All India |
| Certifications | FDCA, GMP, GLP and ISO |
Product Overview
Key Features
Niksan Pharmaceutical provides API and finished formulations of Voglibose in many Indian states of like Uttarakhand, Andhra Pradesh, Kerala, Tamil Nadu, Odisha, Chhattisgarh, Jharkhand, Telangana, Himachal Pradesh, Karnataka, West Bengal, Gujarat, Maharashtra, Delhi, Haryana, Jammu & Kashmir, Punjab, Uttar Pradesh, Assam, Chandigarh, Madhya Pradesh, Bihar, Rajasthan Etc.
Niksan Pharmaceutical is also huge exporter of the API and finished pharmaceutical products of Voglibose in many countries for years. The countries where we exporting are India, Nepal, Japan, United Arab Emirates, Ukraine, Bolivia, Bangladesh, Philippines, Thailand, Saudi Arabia, Italy, Australia, Canada, United States, United Kingdom, Mexico, Indonesia and many other countries.
Voglibose is belongs to drugs class alphaglucosidase inhibitor which used for lowering postprandial blood glucose levels in people with diabetes mellitus. Voglibose delays the absorption of glucose thereby reducing the risk of macro vascular complications.
Voglibose is used for treatment of type II Diabetes Mellitus also used in control blood sugar level in type II Diabetes.
SYNONYMS: Voglibosa, Voglibose, Voglibosum
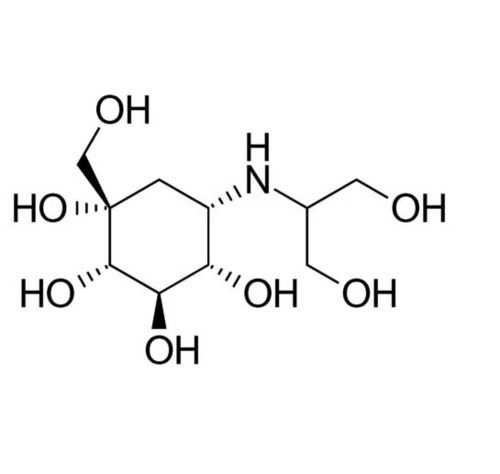
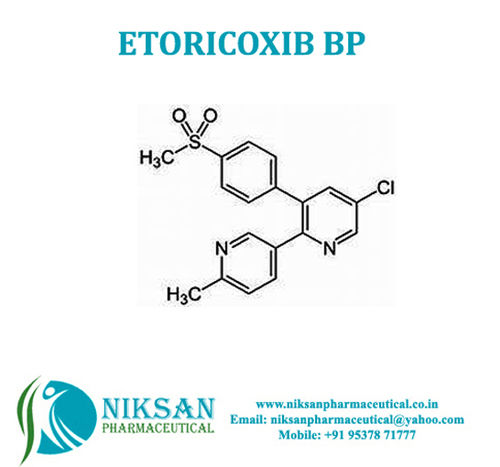
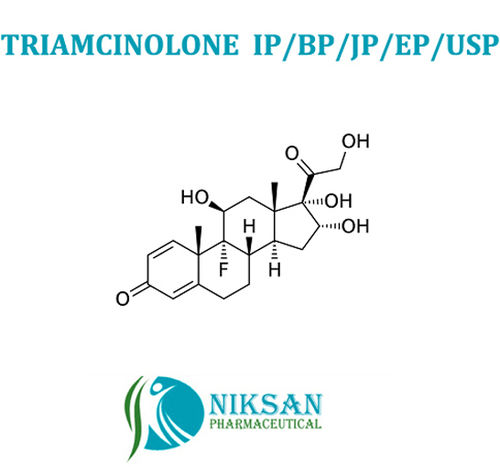
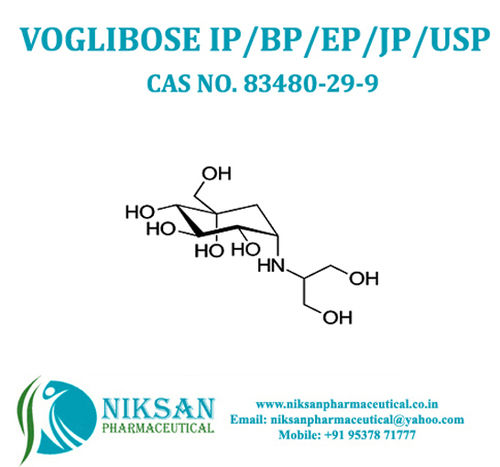
IUPAC NAME: (1S,2S,3R,4S,5S)-5-[(1,3-dihydroxypropan-2-yl)amino]-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetrol.
CAS NO: 83480-29-9
FORMULA: C10H21NO7
MOLECULAR MASS: 267.28 g/mol
STORAGE OF VOGLIBOSE: Store it in cool and dry place, away from moisture and direct light. Keep medicine away from the reach of children and pets. Does not store medicine in bathroom or any humidly place.
APPLICATIONS OF VOGLIBOSE: Voglibose is used to improve blood sugar control in adults with type II diabetes. It is used in the treatment of type II diabetes mellitus. Voglibose is an anti-diabetic medicine. It inhibits the intestinal enzymes responsible for breaking complex sugars like glucose.
HOW TO USE: Voglibose should taken by mouth with or without food.
Follow all directions on your prescription label or as your doctor recommends you.
HOW VOGLIBOSE WORKS: Absorption of glucose delays by Voglibose and also Voglibose cause risk reducing macro vascular complications.
CONTRAINDICATIONS: Do not take Voglibose if you have Diabetic ketoacidosis. Avoid the Voglibose in inflammatory bowed disease, colonic ulcerations and partial intestinal obstructions. Kindly share the information with doctor if you have any chronic disorders of digestion or absorption. Take your doctor advice if you are suffering of Hypersensitivity to the drug or to any of its components.
PHARMACOKINETIKS OF VOGLIBOSE: Absorption is weakly if you take Voglibose by oral administration. Half life of Voglibose is 4.08 hours. Metabolism of Voglibose is minor in liver. The Voglibose excretion is minor and plasma concentrations after oral dose have been undetectable.
SIDE EFFECTS OF VOGLIBOSE: The common side effects of Voglibose are diarrhea, flatulence, Pneumatises Intestinalis, Abnormal liver function, Nausea or vomiting and Dizziness.
PRECAUTION: Inform the doctor if the patient is allergic to the active ingredients and any other drugs like Laparotomy or ileus, Stenos is, Shortness of breath, Severe hepatic Etc.
Do not take Voglibose if you have Roemheld's syndrome.
CDSCO APPROVAL: Voglibose 0.2 mg + Metformin 500mg Tablets are approved by CDSCO in India in 13.07.2009.
Voglibose disp.tab.0.2mg/0.3mg tablets are approved by CDSCO in India in 20.11.2007.
Voglibose0.3 + Metformin (SR) 500mg tablets are approved by CDSCO in India in 06.08.2010.
Voglibose 0.2mg/0.3mg tablet (Mouth dissolving) is approved by CDSCO in India in 28.12.2007.
Voglibose (0.2mg/0.3mg) Tablets are approved by CDSCO in India 17.11.2005.
FORMULATIONS AVAILABLE IN MARKET:
Voglibose 0.3 MG Pellets
Voglibose 0.2 MG Pellets
Voglibose 0.2 mg + Metformin 500mg Tablets
Voglibose disp. tablets0.2mg/0.3mg
Voglibose0.3 + Metformin (SR) 500mg tablets
Voglibose 0.2mg/0.3mg tablets (Mouth dissolving)
Voglibose (0.2mg/0.3mg) Tablets
Glimepiride 2 MG+Metformin 500 MG+Voglibose 0.2 MG tablets
Glimepiride 1 MG+Metformin 500 MG+Voglibose 0.2 MG tablets
Glimepiride 1 MG+Metformin 500 MG+Voglibose 0.3 MG tablets
Repaglinide 0.5 MG+Voglibose 0.3 MG tablets
Repaglinide 1 MG+Voglibose 0.2 MG tablets
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Company Details
NIKSAN PHARMACEUTICAL was established by a group of experienced professionals with an objective to develop Pharmaceutical Products in a complex quality driven atmosphere. we put in our the best efforts to provide Active Pharmaceutical Ingredients and Pharmaceutical Finished Formulation in various dosage forms (Tablets, Capsules, Syrups, Gels, Ointments, Nasal Sprays and Dry powder in pouch Pack) with prime focus on the exports of the APIs and Pharmaceutical Finished Formulation. We maintain high level of integrity and transparency in our business processes and dealings and thus assure high quality products to the customers. We are developing high quality Pharmaceutical Intermediate through Research & Development using new technologies. Our R & D team is expert Process Development, process optimization and scale-up to big quantity.
Business Type
Exporter, Importer, Manufacturer, Distributor, Supplier, Trading Company
Employee Count
18
Establishment
2013
Working Days
Sunday To Monday
GST NO
24AAKFN4352Q1ZR
Payment Mode
Cash Against Delivery (CAD), Cash on Delivery (COD), Cash Advance (CA), Cash in Advance (CID), Cheque, Days after Acceptance (DA), Delivery Point (DP), Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Western Union, Paypal, Others
Certification
FIRM REGISTRATION
Related Products
Seller Details

GST - 24AAKFN4352Q1ZR
Ankleshwar, Gujarat
Sales Department
Mr Sanjay Patel
Members since
12 Years
Address
Plot No. 4706/03, Gidc Estate, SF-12, Shrinathji Arcade, Near Meghmani Chowkadi, Ankleshwar, Gujarat, 393002, India
voglibose in Ankleshwar
Report incorrect details